Bio - GPS
Biomarker Guided Patient Selection
The Kynurenine Pathway

Bio-GPS
Biomarker Guided Patient Selection
BioGPS – Biomarker Guided Patient Selection offers a revolutionary approach to healthcare by tailoring treatments based on individual patient profiles.
How Bio-GPS Works:
- Detection: It begins with identifying and validating relevant biomarkers. This is typically done using advanced genomic, proteomic, or metabolomic techniques. A biomarker is a measurable indicator of a biological state or condition. This can range from levels of specific proteins in the blood, gene mutations, the presence of certain antibodies, or any other measurable biological parameter.
- Classification: Once biomarkers are identified, patients can be classified based on their individual profiles. This might indicate susceptibility to certain diseases, predict disease progression, or even suggest how a disease might respond to treatment.
- Tailored Treatment: Armed with this personalized data, healthcare professionals can select treatments that are more likely to be effective for the individual, and less likely to cause adverse reactions.
Benefits:
- Enhanced Efficacy: By focusing on individual biomarker profiles, treatments can be more directly effective by targeting the root or associated cause of the condition.
- Reduced Side Effects: Minimizing the trial-and-error approach of medicine reduces harmful or unnecessary side effects.
- Cost-Effective: Over the long term, ensuring patients get the right treatment the first time leads to cost savings for both the patient and the healthcare system.
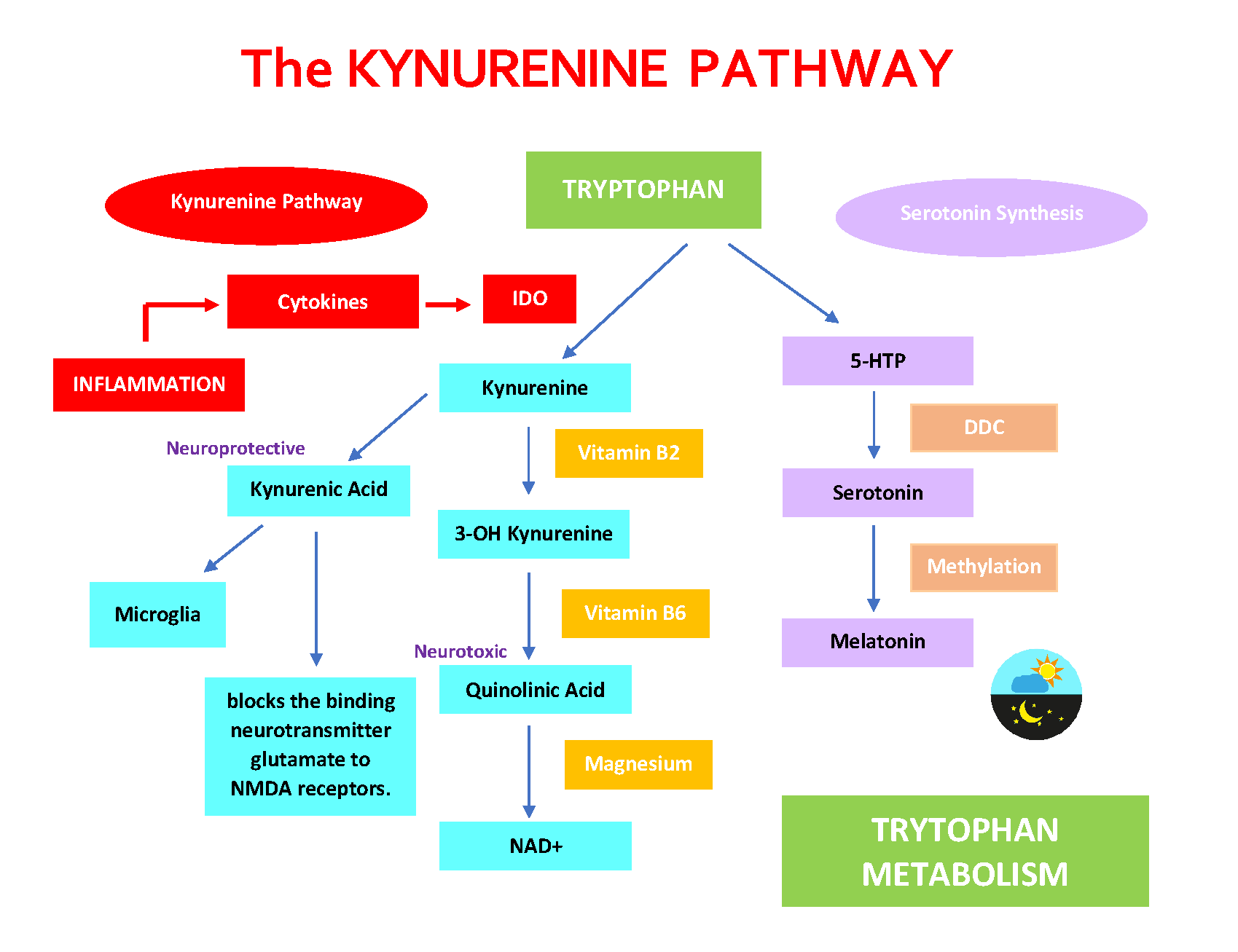
The Kynurenine Pathway (KP)
The Kynurenine Pathway (KP) is the central route for the metabolism of the amino acid tryptophan. It is one of the most researched pathways in the context of both the immune and nervous systems due to its implication in various disease processes.
- Starting Point – Tryptophan: The amino acid tryptophan, which we primarily get from our diet, serves as the precursor for the kynurenine pathway. Only a small portion of dietary tryptophan goes to produce serotonin, while the majority enters the KP.
- Initiation of the Pathway: The conversion of tryptophan to kynurenine is the first and rate-limiting step. This conversion is catalyzed by enzymes such as indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO). The activity of these enzymes can be influenced by various factors, including inflammatory cytokines.
- TDO is mostly liver-specific and is regulated by tryptophan levels, while IDO is found in various tissues and can be induced by inflammatory signals, particularly cytokines.
- Kynurenine Metabolites: Once kynurenine is produced, it can be further metabolized to produce various biologically active compounds, including kynurenic acid, anthranilic acid, 3-hydroxykynurenine, and quinolinic acid. Each of these metabolites has its own set of effects, and their balance can influence neurological and immune functions. For example, kynurenic acid is neuroprotective, while quinolinic acid can be neurotoxic.
- Bioactive Metabolites:
- Kynurenine: The primary metabolite which can be further metabolized into other bioactive compounds.
- Kynurenic Acid: Exhibits neuroprotective effects. It antagonizes the N-methyl-D-aspartate (NMDA) receptor and blocks the α7 nicotinic acetylcholine receptor.
- Quinolinic Acid: Acts as an agonist at the NMDA receptor, leading to excitotoxicity and potentially promoting neuroinflammation.
- Bioactive Metabolites:
- Inflammation and Immune Modulation: Some metabolites of the KP, like kynurenic acid, can act as anti-inflammatory agents by modulating the immune response, including reducing the activity of immune cells. Conversely, other metabolites, such as quinolinic acid, can exacerbate inflammation, promote oxidative stress, and have neurotoxic effects. A shift towards the production of quinolinic acid over kynurenic acid in certain conditions can heighten neuroinflammatory responses and is observed in some neurodegenerative diseases.
- Neurotransmitter Synthesis: The KP is involved in the synthesis of the neurotransmitter serotonin and, indirectly, in the production of the neurotransmitter NAD (nicotinamide adenine dinucleotide), a crucial molecule for various cellular processes.
- Clinical Significance: Dysregulation of the KP has been linked to various conditions such as depression, anxiety, schizophrenia, and neurodegenerative diseases. The pathway is also intricately linked with the immune system and can be influenced by inflammatory processes.
- Role in Immune Regulation: The KP plays a role in immune system modulation. For example, activation of IDO and subsequent metabolism of tryptophan to kynurenine can suppress immune responses, which has implications in cancer, autoimmunity, and infectious diseases.
- Therapeutic Targeting: Given the KP’s involvement in numerous diseases, it has been proposed as a therapeutic target. Drugs that modulate the activity of the pathway’s enzymes or the effects of its metabolites might offer therapeutic benefits for a range of conditions.
The Kynurenine Pathway (KP) And NAD+
The kynurenine pathway (KP) is a critical route for the catabolism of the essential amino acid tryptophan, leading to the production of several metabolites, including nicotinamide adenine dinucleotide (NAD+), a vital coenzyme in cellular energy metabolism.
Here’s how the KP is involved in NAD+ synthesis:
Tryptophan Catabolism: The KP begins with the oxidation of tryptophan, catalyzed by either indoleamine 2,3-dioxygenase (IDO) or tryptophan 2,3-dioxygenase (TDO), producing N-formylkynurenine. N-formylkynurenine is then converted to kynurenine.
Formation of Kynurenine Metabolites: Kynurenine can be metabolized through several pathways, leading to the production of key intermediates such as 3-hydroxykynurenine, anthranilic acid, and kynurenic acid. The pathway splits into several branches, but for NAD+ synthesis, the most relevant branch involves the conversion of kynurenine to 3-hydroxykynurenine by kynurenine monooxygenase (KMO).
Conversion to 3-Hydroxyanthranilic Acid: 3-Hydroxykynurenine is further converted to 3-hydroxyanthranilic acid by the enzyme kynureninase.
Formation of Quinolinic Acid: 3-Hydroxyanthranilic acid is converted to quinolinic acid by the enzyme 3-hydroxyanthranilate 3,4-dioxygenase. Quinolinic acid is a crucial intermediate in the KP and plays a direct role in NAD+ synthesis.
Synthesis of NAD+: Quinolinic acid is converted to nicotinic acid mononucleotide (NAMN) by the enzyme quinolinate phosphoribosyltransferase (QPRT). NAMN is subsequently converted through a series of enzymatic steps to NAD+, involving enzymes such as nicotinic acid mononucleotide adenylyltransferase (NMNAT) and NAD+ synthetase.
Role of NAD+ in Cellular Function
NAD+ is an essential coenzyme involved in numerous cellular processes, including:
- Redox Reactions: NAD+ functions as an electron carrier in redox reactions, essential for ATP production through glycolysis, the citric acid cycle, and oxidative phosphorylation.
- DNA Repair: NAD+ is a substrate for poly(ADP-ribose) polymerases (PARPs), enzymes involved in DNA repair and genomic stability.
- Gene Expression: NAD+ is a substrate for sirtuins, a family of NAD+-dependent deacetylases that regulate gene expression, metabolism, and aging.
- Cell Signaling: NAD+ and its metabolites play roles in cell signaling pathways, including those regulating immune responses and inflammation.
Impact of KP Dysregulation
Dysregulation of the KP affects NAD+ synthesis and contribute to various pathological conditions, including:
- Neurodegenerative Diseases: Imbalances in KP metabolites, such as increased quinolinic acid, leads to neurotoxicity and are implicated in disorders like Alzheimer’s disease and Huntington’s disease.
- Immune Dysfunction: Chronic activation of the KP, often driven by inflammation and immune responses, alter NAD+ metabolism and contribute to immune dysregulation.
- Metabolic Disorders: Alterations in NAD+ levels affect metabolic pathways and contribute to conditions such as diabetes and obesity.
Understanding the kynurenine pathway’s role in NAD+ synthesis is crucial for developing therapeutic strategies aimed at modulating this pathway to treat various diseases and promote healthy aging.
The Kynurenine Pathway (KP), Serotonin Synthesis, Inflammation, and Energy Production
The interplay between the Kynurenine Pathway (KP), Serotonin Synthesis, Inflammation, and Energy Production is complex, and current understanding suggests that disturbances in these processes can have profound implications for health, including neuropsychiatric disorders, fatigue, and other systemic effects. Let’s dive deeper into their interconnectedness:
- Tryptophan Metabolism: Both the KP and serotonin synthesis begin with the amino acid tryptophan. Depending on various factors, tryptophan can either be converted into serotonin or metabolized down the KP.
- Inflammation and KP Activation: Inflammation, especially chronic inflammation, has been shown to stimulate the KP. Inflammatory cytokines, such as interferon-gamma (IFN-γ), upregulate the enzyme indoleamine 2,3-dioxygenase (IDO), which converts tryptophan to kynurenine. As a result, more tryptophan is shunted towards the KP, leading to decreased serotonin synthesis.
- Effects on Neurotransmission: Reduced availability of tryptophan for serotonin synthesis can lead to decreased levels of serotonin, a neurotransmitter associated with mood regulation, appetite, and sleep. Lower serotonin levels have been implicated in depression, anxiety, and other neuropsychiatric disorders.
- KP Metabolites and Neuroinflammation: Some metabolites produced in the KP, especially quinolinic acid, exert neurotoxic effects and contribute to neuroinflammation. Quinolinic acid is an agonist of the NMDA receptor, and its overstimulation can lead to excitotoxicity, potentially damaging neurons.
- Energy Production and Fatigue: Tryptophan and its metabolites, especially those down the KP, influence energy production. Kynurenine and its derivatives can modulate mitochondrial function, which is central to cellular energy production. Disturbances in the KP, therefore, contribute to fatigue or reduced energy production.
- Therapeutic Implications: Recognizing the role of inflammation in shifting tryptophan metabolism can pave the way for therapeutic strategies. For instance, addressing underlying inflammation might help in conditions characterized by fatigue, mood disturbances, or altered energy metabolism.
In Summary: The balance of tryptophan metabolism, involving the KP and serotonin synthesis, is crucial for both brain function and systemic health. Inflammation can shift this balance, potentially leading to neuroinflammation, altered neurotransmission, and disturbances in energy production.
The Kynurenine Pathway (KP) And Serotonin Synthesis
The Kynurenine Pathway (KP) and Serotonin Synthesis are two distinct metabolic pathways for the amino acid tryptophan. The balance between these two pathways has significant implications for an individual’s neurologic and psychiatric health.
- Tryptophan Uptake: Tryptophan, an essential amino acid, is primarily obtained from the diet. Once ingested and absorbed in the intestines, tryptophan can follow one of two major pathways: it can be converted into serotonin or enter the kynurenine pathway.
- Serotonin Synthesis: About 10% of tryptophan is directed towards the serotonin pathway. The conversion of tryptophan to serotonin involves two main steps:
- First, tryptophan is converted to 5-hydroxytryptophan (5-HTP) by the enzyme tryptophan hydroxylase. This is the rate-limiting step in serotonin synthesis.
- Then, 5-HTP is decarboxylated by aromatic L-amino acid decarboxylase (AAAD) to produce serotonin (5-hydroxytryptamine or 5-HT).
- Kynurenine Pathway: The majority of tryptophan (about 90%) is metabolized via the KP. The first and rate-limiting step in this pathway is the conversion of tryptophan to kynurenine. This conversion is catalyzed by the enzymes indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO).
- Interplay and Competition: Both pathways compete for available tryptophan. Factors that upregulate the KP (such as inflammation or stress) can divert tryptophan away from serotonin synthesis, potentially leading to reduced serotonin levels. This is one mechanism by which inflammation or stress can be linked to mood disorders like depression.
- Clinical Implications: Reduced serotonin levels or altered serotonin signaling is implicated in various psychiatric disorders, including depression and anxiety. A shift of tryptophan metabolism toward the KP and away from serotonin synthesis has been proposed as a mechanism in some cases of depression, especially those associated with inflammation.
In summary, the balance between the kynurenine pathway and serotonin synthesis is crucial for mental health. Factors that influence this balance, like inflammation, stress, or genetic predisposition, can impact mood and cognitive function.


